Many women report lowered milk supply ( perceived or actual) and it is a cause of many contacts to breastfeeding experts and sadly a reason many mothers turn to formula when they hadnt intended. Domperidone has for a long time been recommended to help increase supply by utilsising its ability to increase prolactin. It shouldnt be used, in my opinion unless the mother has been supported to achieve regular and effective breastfeeding. It may be needed where a premature baby has been delivered and the mother has been pumping long term – many times the supply starts to dip after 2 weeks.
pdf of this information:
See also UKDILAS information on domperidone and low milk supply https://www.sps.nhs.uk/articles/using-domperidone-for-low-milk-supply
I wrote a factsheet for BfN looking at all the studies on study outcomes some years ago (https://www.breastfeedingnetwork.org.uk/domperidone/ ) . This information is an update on my thoughts and in light of experience and new reports.
Some years ago I wrote a factsheet for the Breastfeeding Network on the use of domperidone as a galactagogue when the recommendations for the use of domperidone changed
https://www.breastfeedingnetwork.org.uk/domperidone/. The MHRA and EHRA recommended that domperidone be prescribed only in limited situations and at a maximum dose of 10mg three times a day for 7 days and not for breastfeeding mothers https://www.gov.uk/drug-safetyupdate/domperidone-risks-of-cardiac-side-effects.
Domperidone has long been used as a galactagogue to increase milk supply. Most of the evidence from studies lies with use to increase lactation after premature delivery but can be used later where breastfeeding got off to a difficult start such as separation of mother and baby. The research studies on domperidone and use to increase milk supply can be found in the BFN link. In my experience it had been used when adequate breastfeeding support had not been given and when feeding was not effective or frequent enough to stimulate supply. This should always be the first intervention and should be happening at every professional contact with a breastfeeding mother.
When should domperidone not be used ?
Domperidone interacts with other medication and should not be used:
- before assessment by someone skilled in breastfeeding support alongside expressing both
breasts, at least 8-10 times in 24 hours including overnight - where either mother or baby has any evidence of cardiac abnormalities and specifically
arrhythmia - where either is receiving other medications known to prolong QT interval or potent CYP3A4
inhibitors e.g., quinolone antibiotics, ketoconazole, fluconazole, macrolide antibiotics, SSRI
antidepressants (risk low with most commonly used ( Funk 2013) tricyclic antidepressants,
salbutamol (https://ggcmedicines.org.uk/media/uploads/ps_extra/pse_21.pdf) - where severe hepatic impairment has been identified in mother or baby
- where either mother or baby has high or low levels of potassium, or low levels of magnesium (https://ggcmedicines.org.uk/media/uploads/ps_extra/pse_21.pdf)
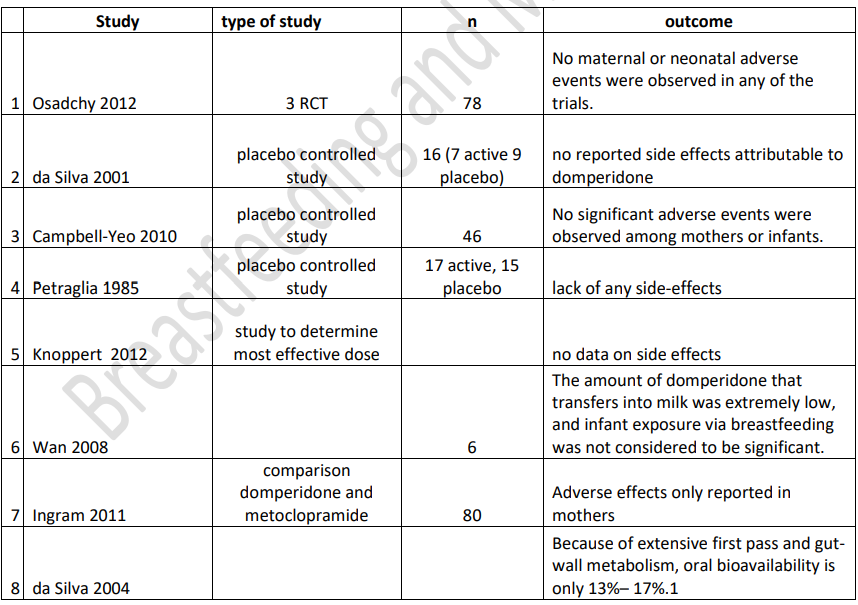
Are there adverse effects of domperidone on babies exposed through their mother’s milk? Hale data(2022) : milk plasma ratio 0.25% (<1 regarded as compatible with breastfeeding), plasma protein binding 93% leaving only 7% which can pass into milk, oral bioavailability 13-17% because of extensive first pass metabolism, relative infant dose 0.01%-0.35% (< 10% regarded as compatible with breastfeeding. Analysis of studies not complete but indicative of low risk to babies of domperidone passing to baby and would be grounds for use in breastfeeding to be reconsidered by EHRA and MHRA.
Higher doses of domperidone to increase milk supply?
Some breastfeeding experts have used significantly higher doses of domperidone to stimulate
lactation and continued use long term without reported adverse effects (Newman J, Polokova A What Doctors Don’t Know About Breastfeeding 2022)
Tapering off dose
There are no studies that provide an evidence base on how long to continue domperidone in the case of inadequate lactation (Academy of Breastfeeding Medicine (ABM) 2018 Anecdotally, some women feel that their supply cannot be maintained without the drug, while some can reduce the dose but not stop altogether. It is possible that domperidone is acting as a placebo to boost their confidence – we do not know and should admit the limitations of the research.
After a slow withdrawal from domperidone, one study found no significant increase in formula
supplementation suggesting that once sufficient milk production is established, it is maintained even without the use of domperidone (Livingston 2007).
Knoppert (2012) showed that in 3 out of 4 women who had taken domperidone for 4 weeks at full dose, 2 weeks at reducing dose, milk supply was maintained. Although gradual weaning from the drug has become standard, there is little published evidence apart from the reports and data is based on the experience of breastfeeding specialists.
Withdrawal
Drug withdrawal symptoms consisting of insomnia, anxiety, and tachycardia were reported in a woman taking 80 mg of domperidone daily for 8 months as a galactogogue who abruptly tapered the dose over 3 days (Papastergiou 2013).
Another mother (Seeman2015) took domperidone 10 mg three times daily for 10 months as a galactagogue and stopped abruptly. After discontinuation, she experienced severe insomnia, severe anxiety, severe cognitive problems and depression.
A third postpartum woman (Doyle 2018) began domperidone 90 mg daily, increasing to 160 mg daily to increase her milk supply. Because her milk supply did not improve, she stopped nursing at 14 weeks and began to taper the domperidone dosage by 10 mg every 3 to 4 days. Seven days after discontinuing domperidone, she began experiencing insomnia, rigors, severe psychomotor agitation, and panic attacks. She restarted the drug at 90 mg daily and tapered the dose by 10 mg daily each week. At a dose of 20 mg daily, the same symptoms recurred. She required sertraline, clonazepam and reinstitution of domperidone at 40 mg daily, slowly tapering the dose over 8 weeks. Three months were required to fully resolve her symptoms.
In a fourth case (Manzouri 2017), a mother took domperidone 20 mg four times daily for 9 months to stimulate breastmilk production. She stopped breastfeeding and domperidone at that time. Two weeks later, she presented with insomnia, anxiety, nausea, headaches and palpitations. The drug was restarted at a dosage of 20 mg three times daily and began to taper the daily dosage by 10 mg every week, but after one week she complained of insomnia. Tapering was reduced to 5 mg every week, but whenever she stopped the ndrug, symptoms returned. She was able to discontinue domperidone after tapering the daily dosage by 2.5 mg weekly over 10 months A fifth case (Sharma 2022) of a mother with a history of bipolar disorder and major depression developed severe anxiety, a recurrence of depression and obsessive compulsive disorder 6 days after abruptly discontinuing domperidone 120 mg daily that she was aking as a galactogogue. Three days later, she restarted domperidone 120 mg daily and tapered her daily dose by 10 mg at weekly intervals. She took no other medications. Two weeks after discontinuing domperidone, she had signs of only mild mood disorder. Hale (2022) reports an increase in calls to InfantRisk reporting problems with withdrawal after high dose and long-term use.
This should in my opinion be discussed with the mother before prescription of the medication.
References
- Academy of Breastfeeding Medicine ABM Clinical Protocol #9: Use of Galactogogues in
Initiating or Augmenting Maternal Milk Production, Second Revision Bodribb W 2018.
Breastfeed Med. 2018 Jun;13(5):307-314 - Asztalos EV, Kiss A, daSilva OP, Campbell-Yeo M, Ito S, Knoppert D; EMPOWER Study
Collaborative Group. Evaluating the Effect of a 14-Day Course of Domperidone on Breast
Milk Production: A Per-Protocol Analysis from the EMPOWER Trial. Breastfeed Med. 2019
Mar;14(2):102-107. doi: 10.1089/bfm.2018.0175. Epub 2018 Dec 13. PMID: 30543461. - Campbell-Yeo ML, Allen AC, Joseph KS et al. (2010) Effect of domperidone on the
composition of preterm human breast milk. Pediatrics 125(1): e107-114. - da Silva OP, Knoppert DC, Angelini MM et al. (2001) Effect of domperidone on milk production in mothers of premature newborns: a randomized, double-blind, placebo-controlled
trial. CMAJ 164(1): 17-21 - da Silva OP, Knoppert DC. (2004) Domperidone for lactating women. CMAJ 171(7): 725
- Doyle M, Grossman M. Case report: domperidone use as a galactagogue resulting in
withdrawal symptoms upon discontinuation. Arch Womens Ment Health. 2018
Aug;21(4):461-463. doi: 10.1007/s00737-017-0796-8. Epub 2017 Oct 31. PMID: 29090362. - Funk KA, Bostwick JR. A comparison of the risk of QT prolongation among SSRIs. Ann
Pharmacother. 2013 Oct;47(10):1330-41) - Grzeskowiak LE, Smithers LG, Amir LH, Grivell RM. Domperidone for increasing breast milk
volume in mothers expressing breast milk for their preterm infants: a systematic review and
meta-analysis. BJOG. 2018 Oct;125(11):1371-1378. doi: 10.1111/1471-0528.15177. Epub
2018 Mar 27. PMID: 29469929. - Grzeskowiak LE, Wlodek ME, Geddes DT. What Evidence Do We Have for Pharmaceutical
Galactagogues in the Treatment of Lactation Insufficiency?-A Narrative Review. Nutrients.
2019 Apr 28;11(5):974. doi: 10.3390/nu11050974. PMID: 31035376; PMCID: PMC6567188. - Hale TW and Krutsch K Medications and Mothers Milk 2022 Springer Publications
- Ingram J, Taylor H, Churchill C, Pike A, Greenwood R, Metoclopramide or domperidone for
increasing maternal breast milk output: a randomised controlled trial Arch Dis Child Fetal
Neonatal Ed F2 of 5(2011). doi:10.1136/archdischild-2011-300601 - Knoppert DC, Page A, Warren J, Carr M, Angelini M, Killick D, DaSilva OP, The Effect of Two
Different Domperidone Doses on Maternal Milk Production published online 3 May 2012 J
Hum Lact - Livingston V, Blaga Stancheva L, Stringer J. The effect of withdrawing domperidone on
formula supplementation. Breastfeeding Med. 2007; 2:278, Abstract 3.